Amazon policy about laser product compliance requirements:
Please see below for the policy detail information of the Amazon about the sale of laser products, since November 11, 2019, the sale of the Class IIIB and Class IV laser products without the FDA authorization are forbidden on Amazon America.
Amazon has a security policy for the following products:
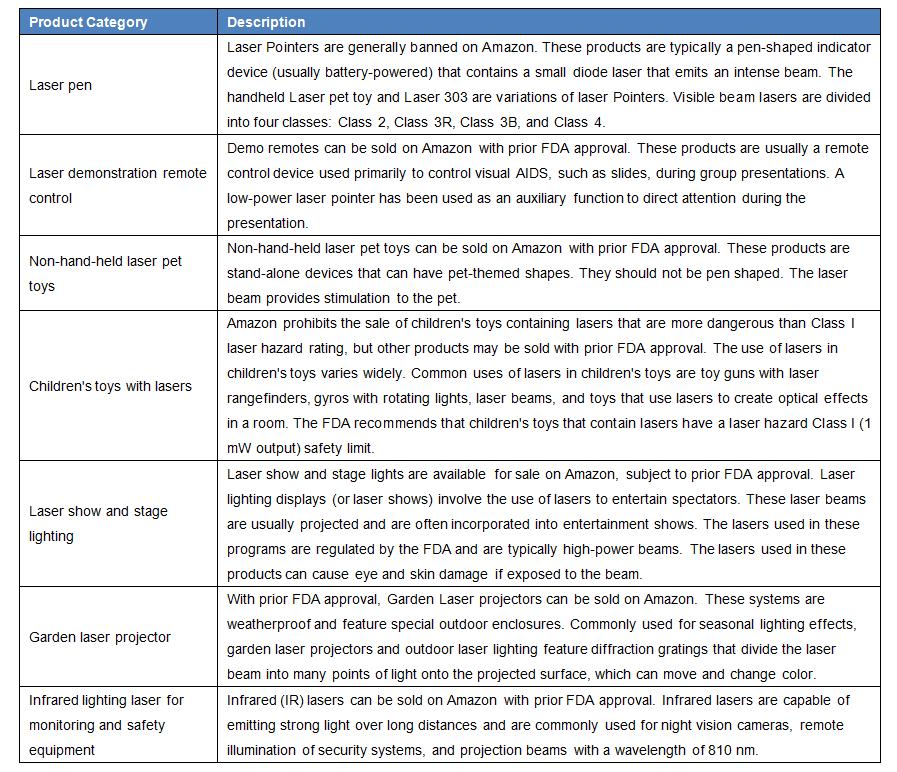
Laser product that is pre-FDA approved for sale:
In order to sell laser products on Amazon, you must apply by sending the following information to the email address listed in the table below
• Company Name
• Seller No.
• E-mail Address
• Telephone Number
• The ASIN list of laser products you apply to sell
• Food and Drug Administration (FDA) Accession Letter for each ASIN you applied to sell
• Documentation from a National Recognized Testing Laboratory (NRTL) proving that your product has been tested in accordance with the standards listed in the table below
The laser products are required for compliance certification:
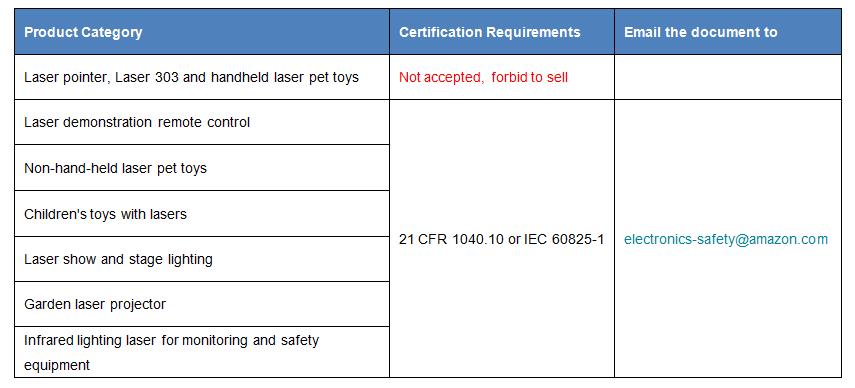
FDA certification of the SPECIMEN laser product:
21 CFR 1040.10 or IEC 60825-1,or submit FDA Product Report for IEC 62471-5 new model of laser projector to obtain FDA Accession letter and FDA Accession Number, each year on September 1, before the annual report submitted to the FDA to maintain the effectiveness of the FDA certification, not only the sample requested by the FDA to need to meet the 21CFR1020/21CFR1030/21CFR1040 federal regulatory requirements, and asked each of the products exported to the United States must conform to the requirements of federal regulations, Therefore, FDA factories need to establish a sound and complete IQC inspection of incoming materials, production process inspection, pre-shipment inspection and other whole process product quality control system.
Summary of FDA CDRH certification standards for radiation products:
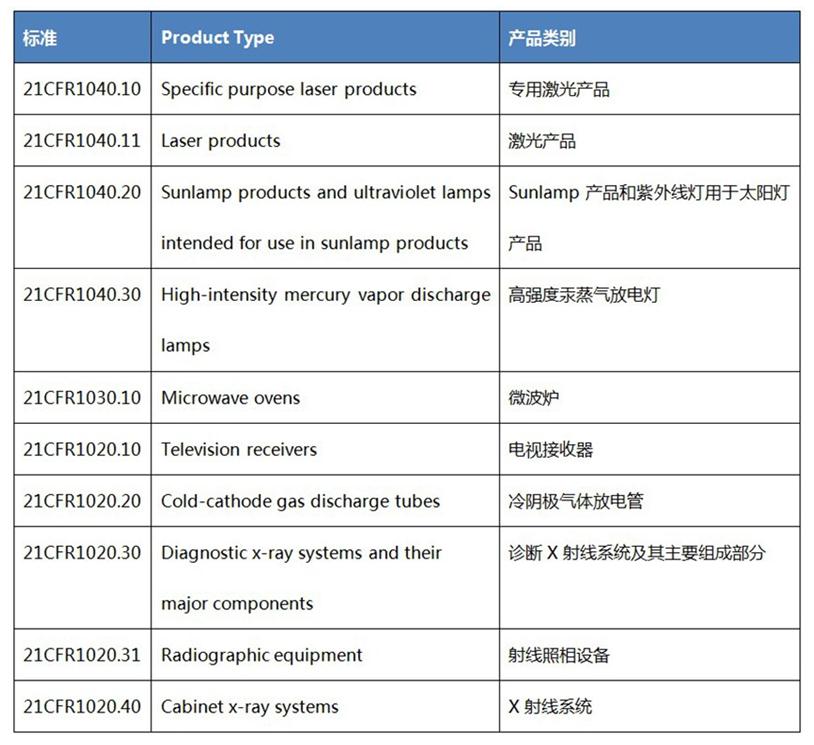
What is the FDA?
The U.S. Food and Drug Administration (FDA), part of the U.S. Department of Health, Education and Welfare, is responsible for the national regulation of drugs, Food, biological products, cosmetics, veterinary drugs, medical devices, and diagnostic supplies. The Center for Devices and Radiological Health (CDRH) is a division of the FDA that provides timely, consistent, safe, and effective, high-quality medical Devices and safe radiation-emitting products to protect and promote public Health.
What products are subject to mandatory FDA certification?
The FDA generally requires all LED products to be included in Federal regulations 21 CFR 1000-1005 and must be declared to the FDA. However, LED lighting products that emit visible light, such as common LED lights, are not covered by the mandatory FDA certification of 21 CFR 1000.3. Therefore, there is no need to submit FDA product report and annual report for the import of ordinary LED lights into the US market, and there is no need to implement FDA certification performance standards. Currently, the products under the mandatory control of FDA radio-emitting electronic products include Laser, X-ray, Microwave and Microwave products, which have specific FDA specifications.
What is the FDA-2877 form?
The FDA-2877 FORM of the United States is "Declaration on the Compliance of Imported Electronic Products with Radiation Control Standards", for the full English name, please see the following declaration form:
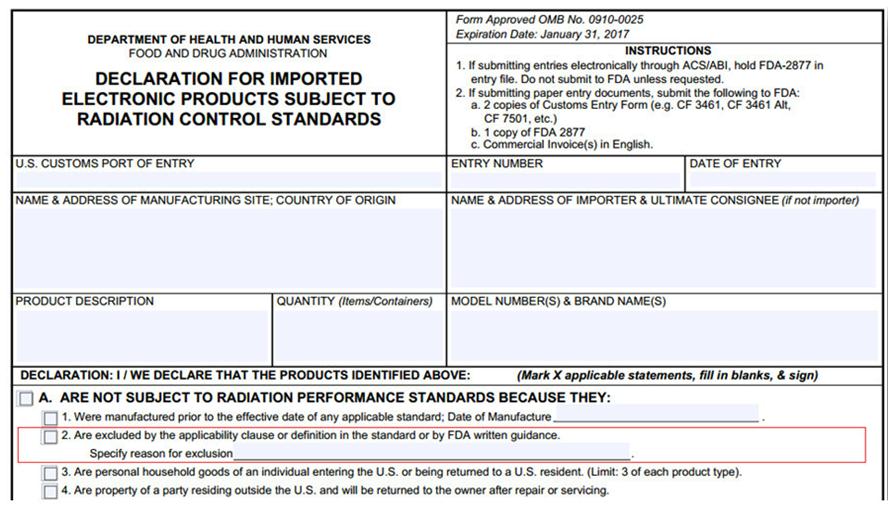
Official link to the original FDA-2877 form:https://www.fda.gov/downloads/aboutfda/reportsmanualsforms/forms/ucm080778.pdf
For more information about Amazon's policies, compliance requirements and FDA certification, please feel free to contact BACL technical experts. We will confirm with the official and give you a timely and accurate reply to ensure that your products enter the North American market smoothly!
BACL Service:
BACL is a North American safety certification body accredited by American OSHA & Canada SCC, IECEE CB Scheme NCB and CBTL(CB102, TL730, TL792, TL796, TL360 ), and EU CAB(Conformity Assessment Body) ( Notified Body No.1313). At the same time BACL is authorized and recognized by A2LA, IAS, CNAS, CMA, FDA, ACMA, SAA etc, and sets its safety testing labs in US, South Korea, Taiwan, Shenzhen Futian & Nanshan, Dongguan, Chengdu, Kunshan.
We can provide you with global certification of IT/AV, HOUS, lighting products, power products, electric tools and other products. Certification service includes US (NRTL, FCC, ENERGY STAR, FDA, DOE, CEC) , Canada(SCC, ISED, NRCan), EU(CE), Korea(KC), EU CE NB, IECEE CB Scheme, Japan (MIC), Singapore (IMDA), Hong Kong (OFCA), Taiwan(NCC, BSMI), Egypt (NTRA, GOEIC), Vietnam (MIC), Saudi Arabia (SASO), Philippines (NTC), Thailand (NBTC), Malaysia (SIRIM), India (BIS/WPC/TEC) and other countries’ certification services, ensuring that your products can enter the international market smoothly under increasingly fierce international trade barriers.

 CN/中国
CN/中国  US/USA
US/USA  KR/Korea
KR/Korea  DE/Germany
DE/Germany  ES/Spain
ES/Spain VN/Việtnam
VN/Việtnam